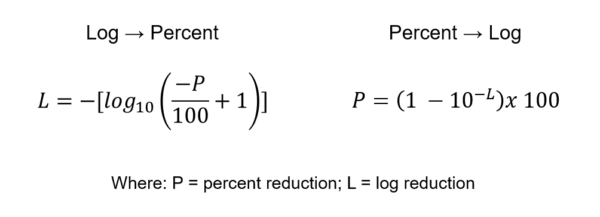BIOSAFE® organosilanes antimicrobials provides long-lasting surface protection against bacteria, fungi, mold, mildew, and algae.
BIOSAFE® is a silicon-based antimicrobial that imparts bacteriostatic, fungistatic, and algistatic properties to coatings and resins for manufactured goods. It is available as a powder, solvent or aqueous solutions. With proper integration, BIOSAFE® products have high antimicrobial performance in ISO and ASTM testing. The active ingredient in the BIOSAFE® Antimicrobial was developed and registered with the EPA in the mid 1970s, and has since been widely utilized in numerous applications for over 35 years. BIOSAFE® has expanded the breadth of applications and manufacturing processes by removing the VOCs and making it available in a new polymeric powder. This powder, similar to its solvent-based predecessors, can be formulated into coatings, but unlike its predecessors, the powder can be compounded with polymer resins.
The antimicrobial, when properly incorporated in accordance with the instructions and specifications provided, imparts a protective bacteriostatic, fungistatic and algistatic surface on substrates to prevent deterioration and discoloration caused by fungi, prevent algae growth and inhibits the growth of odor-causing bacteria. It is durable, leach resistant, and non-migrating. It does not create the conditions that promotes the development of resistant microorganism.
The active ingredient in BIOSAFE® forms a colorless, odorless, positively charged polymer that molecularly bonds to your product’s surface. The strong positive charge disrupts the cell membrane of all microorganisms that come in contact with the active surface, thereby causing their cytoplasm and organelles to leak out. It resembles popping a water balloon. Because of this physical kill mechanism, BIOSAFE® does not promote the development of drug resistant superbugs.
The demand for antimicrobial agents is on a steady rise and will continue to grow in the coming years. The conventional antimicrobial agents are silver based or triclosan based, each of which have their own disadvantages either in terms of cost or leaching from the treated surfaces and ending up in the water stream.
BIOSAFE® is a new class of anti-microbial which is derived from organo- functional silanes. Not only does BIOSAFE® have the anti-microbial properties, but it also has the unique properties of a silane to be able to covalently bond to a substrate.
BIOSAFE® in its powder form can also be used as an additive in coating formulations or compounded with plastics to create materials that are inherently anti-microbial. The ability to covalently bond to a substrate prevents Biosafe® from leaching.
BIOSAFE® does not display any color change which is a common problem with the silver based antimicrobial agents. Details on the wide range efficacy of action for BIOSAFE® will be presented along with its comparison to the most commonly used antimicrobial agents.
A microbe’s only protection from the outside world, the cell wall, is about 20 nanometers thick. 1/1000th the thickness of a plastic grocery bag, thin and vulnerable.
Yet most antimicrobial technologies are designed to pass right through the cell wall and attempt to poison the microbe from the inside, interfering with its metabolism and altering its DNA. This leads to mutations and resistant-adaptive superbugs! What a bad idea! Silver and triclosan, both chemicals that operate in this manner, are technologies that have increasing health and safety concerns globally, both for toxicity and mutation and adaptation of super-bugs.
BIOSAFE® is not metabolized by cells, it creates a network of charged particles that pop cells like a balloon, on contact. The technology takes advantage of the weakness of the cell wall and ruptures it with this physical/electrical force. BIOSAFE®’s invisible coating adheres to hard surfaces and fabrics, and is ready to destroy any microbes that come in contact with it. What’s more, it provides durable, long lasting protection for 30 days. This physical rupturing of the cell wall is the best way to kill a microbe. It does not lead to resistant superbugs, and the active is not leached into the environment or person, as occurs with silver and triclosan technologies. Antimicrobials are not all created equal. BIOSAFE® is the only antimicrobial polymer in its class that provides unmatched safety and performance.
BIOSAFE®’s powder and water based solutions are registered with EPA’s Antimicrobials division as EPA Reg. No. 83019-1,2,3
Antimicrobial additives for surfaces and textiles perform differently and are regulated differently than sanitizers or disinfectants. Antimicrobial “additives” are considered to be microbiostatic agents that protect products, not people, per EPA regulations. The key distinction is that these are non-health claims. BIOSAFE® is used to protect any material or textile, under its approved uses, to mitigate or stop the growth of product damaging microbes. Key considerations regarding the end-product’s performance include its environment, the wearability of the surface, and the degree of microbial control needed to achieve the product’s purpose.
When deciding to use an antimicrobial additive, the first thing to consider is the required product performance and how marketing claims will communicate that performance. is is the common starting point for all antimicrobial products. The formula used for a product controlling algae in water lines will differ from the formula controlling either odor-causing bacteria in a refrigerator gasket or damaging mold and mildew in building materials. The activity of a microbiostatic treatment on a bulk surface or textile, such as BIOSAFE®, are verified by standardized industry methods.
Test methods commonly used to determine the antimicrobial activity of solid treated articles are:
- JIS Z 2801 – a method where microbes are inoculated onto an antimicrobial surface and covered for 24hrs, and survivors are counted.
- ASTM E2149 – a method where the material is immersed and shaken in a microbial solution for 1hr, then removed, and surviving organisms are counted.
Third party verification is available from labs that perform this type of “treated article” testing. Please contact us for recommendations of independent third party labs that are EPA and FDA compliant.
When reviewing results from microbiological studies it is important to understand the definitions of the terms and scope of the numbers. Results are reported in either percent reduction or scientific notation.
- 1 log reduction = 90% reduction
- 2 log reduction = 99% reduction
- 3 log reduction = 99.9% reduction
- 4 log reduction = 99.99% reduction
- 5 log reduction = 99.999% reduction
- 6 log reduction = 99.9999% reduction
So, if reviewing a study report from a lab indicating a 3.5 log reduction, then you know it corresponds to a percent reduction somewhere between 99.9% and 99.99% but a 2.5 log reduction does not equal a 99.5% reduction.
These tests were conducted under GLP protocol
- Cytotoxicity: Agar Diffusion Assay ISO 10993-5
- Acute Dermal Toxicity OPPTS No. 870.1200
- Acute Dermal Irritation OPPTS No. 870.2500
- Acute Eye Irritation OPPTS 870.240
- Acute Oral Toxicity Study (USP) OPPTS 870.1100
- Acute Inhalation Toxicity Study OPPTS 870.130
- Skin Sensitization: OPPTS 870.2600
- HRIPT- Human Repeat Insult Patch Test: 48 day
- AMES- Mutagenicity Study
*all studies are available for review upon request
Does this product have EPA registration?
Yes, the active ingredient in BIOSAFE® is a patented and EPA registered siloxane polymer EPA Reg. No. 83019-1-4. Coating and treated articles themselves are exempt from registration under 40 CFR 152.25(a).
How has the BIOSAFE® been tested?
The antimicrobial effectiveness and safety has been verified by independent GLP/EPA registered labs:
- Antimicrobial Effectiveness
ISO 22196:2007
ASTM E-2149-10 - Safety Studies
USP Class VI
ISO 10993-1
EPA Acute Toxicity Package
BIOSAFE’s antimicrobial effectiveness is verified by ISO and ASTM standards as related to the product and its use.
What can I apply BIOSAFE® into/onto?
BIOSAFE® can be incorporated into or applied on to any hard, non-porous surface, any textile, upholstery, or soft porous good. It’s ideal for bulk surfaces and for fabrics that are susceptible to microbial contamination.
How does the BIOSAFE® technology work?
The active ingredient in BIOSAFE® forms a colorless, odorless, positively charged polymer that molecularly bonds to your product’s surface. The strong positive charge disrupts the cell membrane of all microorganisms that come in contact with the active surface, thereby causing their cytoplasm and organelles to leak out. It resembles popping a water balloon. Because of this physical kill mechanism, BIOSAFE does not promote the development of drug resistant superbugs.
BIOSAFE® and other microbiostatic treated products fall under the jurisdiction of EPA and the “treated article exemption”. Before production, a manufacturer must understand the regulatory limitations of a treated object, textile, or liquid as well as the limitations of possible marketing claims. For instance, a yoga mat manufacturer can state the mat is composed of a material that prevents the growth of odor causing microbes and be within regulatory limitations. However, if a yoga mat manufacturer stated that the mat kills 99% of MRSA, then the manufacturer is making a marketing claim that is considered a health claim, which is outside the scope of a microbiostatic agent. Products incorporating BIOSAFE® into or onto the surface are operating under the EPA’s “treated article exemption”. The “treated article exemption” removes the necessity for manufacturers using BIOSAFE® interface with EPA, provided the manufacturers use BIOSAFE® in accordance with the EPA label and the claims center on the protection of the product, not the protection of people. Please Note: EPA regulations can be somewhat nuanced and the interpretation of the rules may change depending on EPA staff perspective. Thus, Gelest encourages verifying regulatory compliance with EPA or EPA consultants prior to sale of their BIOSAFE® containing product.
| Product | Description | EPA Registration # | CAS # | Application |
|---|---|---|---|---|
| HM4100 ANTIMICROBIAL | 84% dry powder | 83019-1 | 199111-50-7 (trihydroxy) | Resin blending and compounding |
| HM4005 ANTIMICROBIAL | 5% in water | 83019-3 | 199111-50-7 (trihydroxy) | Water-based surface treatments |
| HM4001 RTU ANTIMICROBIAL | 0.75% in water | 83019-2 | 199111-50-7 (trihydroxy) | Water-based surface treatments |
| HM4072 ANTIMICROBIAL | 72% in methanol | 83019-4 | 27668-52-6 (trimethoxy) | Water-based surface treatments |
| HE4005 ANTIMICROBIAL | 5% in water | 83019-5 | 199111-50-7 (trihydroxy) | Water-based surface treatments Methanol free! |
| HE4001 ANTIMICROBIAL | 0.75% in water | 83019-6 | 199111-50-7 (trihydroxy) | Water-based surface treatments Methanol free! |
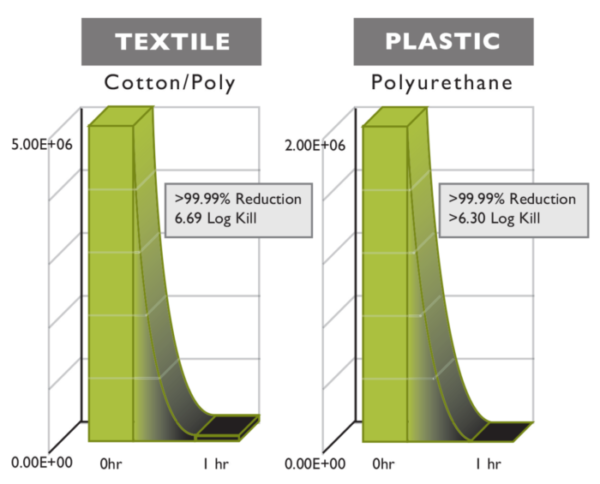
Products with BIOSAFE® properly integrated have high antimicrobial performance in ISO and ASTM testing. Typically, when a product performs with 3-logs (99.9%) or greater it will exhibit bacteriostatic, fungistatic, and algistatic properties in the finished good. Most antimicrobial technologies are designed to pass through the cell wall and attempt to poison the microbe from the inside by interfering with the microbe’s metabolism and altering the microbe’s DNA. This may lead to mutation and adaptive microorganisms, i.e. resistant superbugs.
BIOSAFE® is not metabolized by the microbial cells, instead it creates a network of electrically charged molecules on the surface, which rupture the cell wall on contact. BIOSAFE® creates an invisible coating which bonds to the applied surface and is ready to destroy any microbes that come in contact with the surface. This physical rupturing of the cell wall is a superior way to kill a microbe. It does not promote resistant superbugs and it is not actively leached into the environment or person, as observed with silver or triclosan technologies.
Making choices about which antimicrobial technology to use for your product is not easy. Leaching antimicrobials may claim to be “bound”, “embedded”, or “contained” but they are designed to diffuse and migrate, and therefore are not “chemically bound”. It’s important to understand the facts about modes of action, safety and handling, leaching, and color before choosing your antimicrobial.
| BIOSAFE Silane-Based | Silver-Based | Triclosan-Based | |
|---|---|---|---|
| Mode of Action | Physically ruptures the cell membrane | Releases ionic free radicals that react with cell DNA and disrupt critical life processes in the cell | Releases chlorinated phenols and other organic radicals for consumptions or cellular absorption, causing lethal mutations |
| Leaching or Non-leaching | Chemically bound, covalent bonding, and non-leaching | Embedded and designed to leach out and be metabolized by cells | Embedded and designed to leach out and be metabolized by cells |
| Cost In-use | Economical | Expensive | Moderate |
| Adaptive Organisms | Does not promotes adaptive organisms | Can create adaptive organisms | Can create adaptive organisms |
| In-Plant Safety and Handling | Mild eye irritation | Harmful if inhaled Harmful if absorbed through skin Moderate eye irritation | Harmful if absorbed through skin Moderate eye irritation Avoid contact with skin, eyes, and clothing Do not breathe dust |
| Color in Polymers | Does not change product color | Color shifts to brown, gray, and yellow can occur | Does not change product color |
The polymeric antimicrobial powder HM4100 can be dry blended into the bulk resin by any variety of standard mixing operations. The powder will adhere to the surface of the bulk resin particles and become homogeneously mixed. Following mixing the HM4100 into the host resin, it is ready for molding, extrusion, or other thermoplastic processes to make the finished good. Care should be taken to keep this mixture in a dry environment. In certain resins it is recommended that the mixture be dried before it is used. HM4100 is hygroscopic and can hold 5% moisture.
Resin Compatibility
- TPU- thermoplastic Polyurethane
- Urethane Foam
- Polyether block amide (PEBAX®)
- Polyamide 6 (PA6 or nylon 6)
- Silicone (not post-cured)
- Acrylic (cast and thermoplastic)
- Acrylic adhesives
- Spun fiber (PA6, PE, PP)
- Fiberglass Gel Coat
Loading Level
Dependent upon the finished goods requirement for efficacy, one can use between 1 part per thousand to 10 parts per thousand of BIOSAFE® to base resin by weight (0.1%- 1.0%). BIOSAFE® is dispersed throughout the polymer matrix via blending followed by polymer processing (i.e. thermoplastic, thermoset, etc.).
Temperature
A process temperature of < 170 °C is preferred, but it is time, temperature, and aerobic dependent. BIOSAFE® can survive short times at temperatures up to 250 °C if there is little to no oxygen present, as in injection molding or fiber spinning.
Time, Oxygen, and Heat History
BIOSAFE® is vulnerable to thermal breakdown: A thermal gravimetric analysis shows the product is stable up to about 250 °C in an inert (i.e. Nitrogen) oxygen-free and water-free environment. In commercial use, time, temperature, and chemical reactivity will affect the stability of BIOSAFE®. Additionally the number of heat histories should be minimized. It is preferable to feed the product into an extruder downstream nearest to the die, rather than masterbatch and go through two full heat histories.
Applying BIOSAFE® to surfaces can be accomplished by the following sequence: Typical substrate candidates include carpet, apparel, non-wovens, roll goods, building materials, shoes, and indoor environment surfaces.
Agitation
Under constant, mild agitation, pour the required amount of concentrated HM4072 or HM4005 Antimicrobial into a container with a previously determined volume of water.
Temperature, pH, and Hardness
At a temperature of about 22 °C (75 °F), and a pH between 4.5 and 6.5, water should be soft to slightly hard. Upon mixing with water, the antimicrobial is then ready to react with the substrate.
Anionic Residue
It is essential that the antimicrobial finish be applied to a substrate that is clean. CAUTION! Anionic detergents should not be used. Because the antimicrobial functions via its cationic charge, excessive anionic material in the presence of the antimicrobial will decrease its efficacy.
Concentration
The EPA authorized limits of final application concentrations are from 0.01 to 1.0% of active based upon substrate weight. Most applications are optimized at approximately 0.25-0.5%. Before commercialization, one should thoroughly test any application; optimize concentrations, and independently investigate satisfactory performance. Furthermore, when used in combination with other chemicals (i.e. finishes) testing should be performed to demonstrate proper treatment and compatibility.
- Comparison of the Bacterial Removal Performance of Silver Nanoparticles and a Polymer Based Quaternary Amine Functiaonalized Silsesquioxane Coated Point-of-Use Ceramic Water Filters
- New Antimicrobially Amended Media for Improved Nonpoint Source Bacterial Pollution Treatment
- Computer Keyboard Covers Impregnated with a Novel Antimicrobial Polymer Significantly Reduce Microbial Contamination – Journal
- Gelest – Advancing Antimicrobial Technology To Protect Medical Textiles